This site is intended for healthcare
professionals in Belgium and Luxembourg.

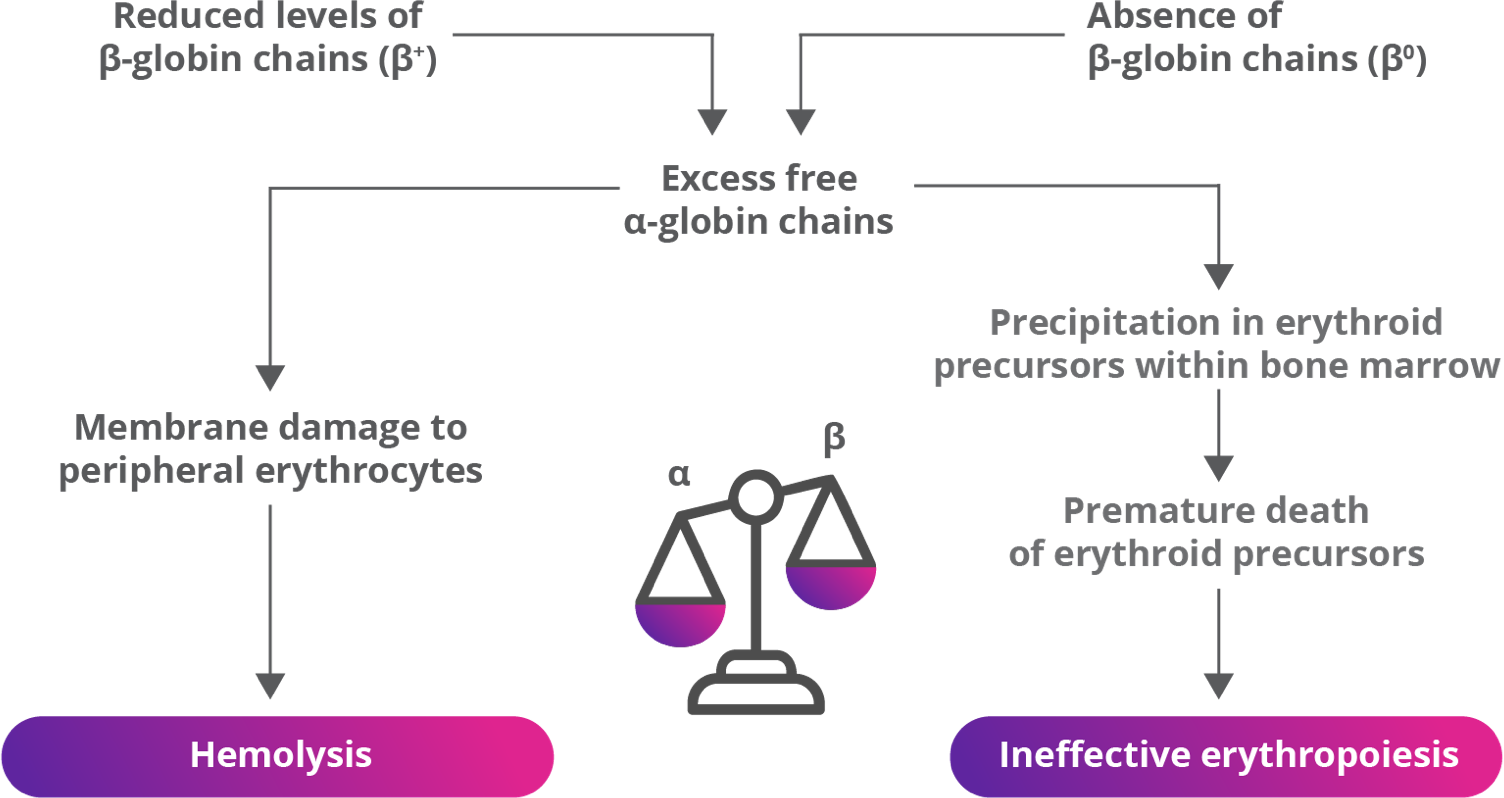
Ineffective erythropoiesis due to mutations in the β-globin gene and hemolysis are hallmarks of
β-thalassemia.1-3
β-thalassemia is a rare genetic condition that reduces production of hemoglobin (Hb). It is characterized by reduced or absent synthesis of the β-globin chain of Hb, decreased Hb in the blood, reduced RBC production and anemia.2,4,5
β-globin gene defects lead to reduced (β+) or absent (β0) synthesis of the β chains of hemoglobin and affected patients can have various combinations of normal (β), β+, and/or β0 alleles.2,4,5
β-thalassemia is caused by mutations in the β-globin gene,
resulting in ineffective erythropoiesis2,4,5
An estimated 80 million to 90 million people—approximately 1.5% of the global population—are carriers of a β-thalassemia mutation.6
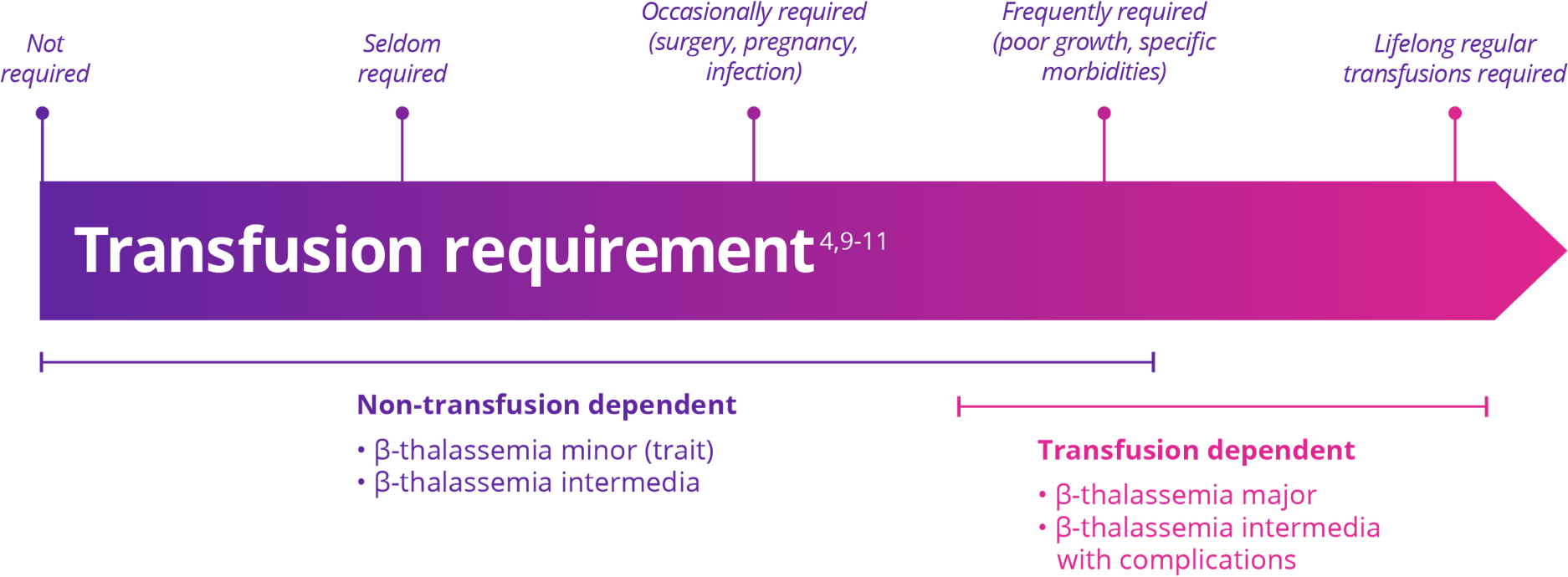
Approximately 200 disease-causing mutations in the β-globin gene have been documented.6 Traditionally, β-thalassemia was classified into 3 main subgroups8,9:
Patients are classified as either transfusion dependent or non-transfusion dependent based on the need for transfusions in order to survive5,9
Current therapy of β-thalassemia is predominantly limited to lifelong red blood cell (RBC) transfusions plus iron chelation therapy (ICT).2,11,12
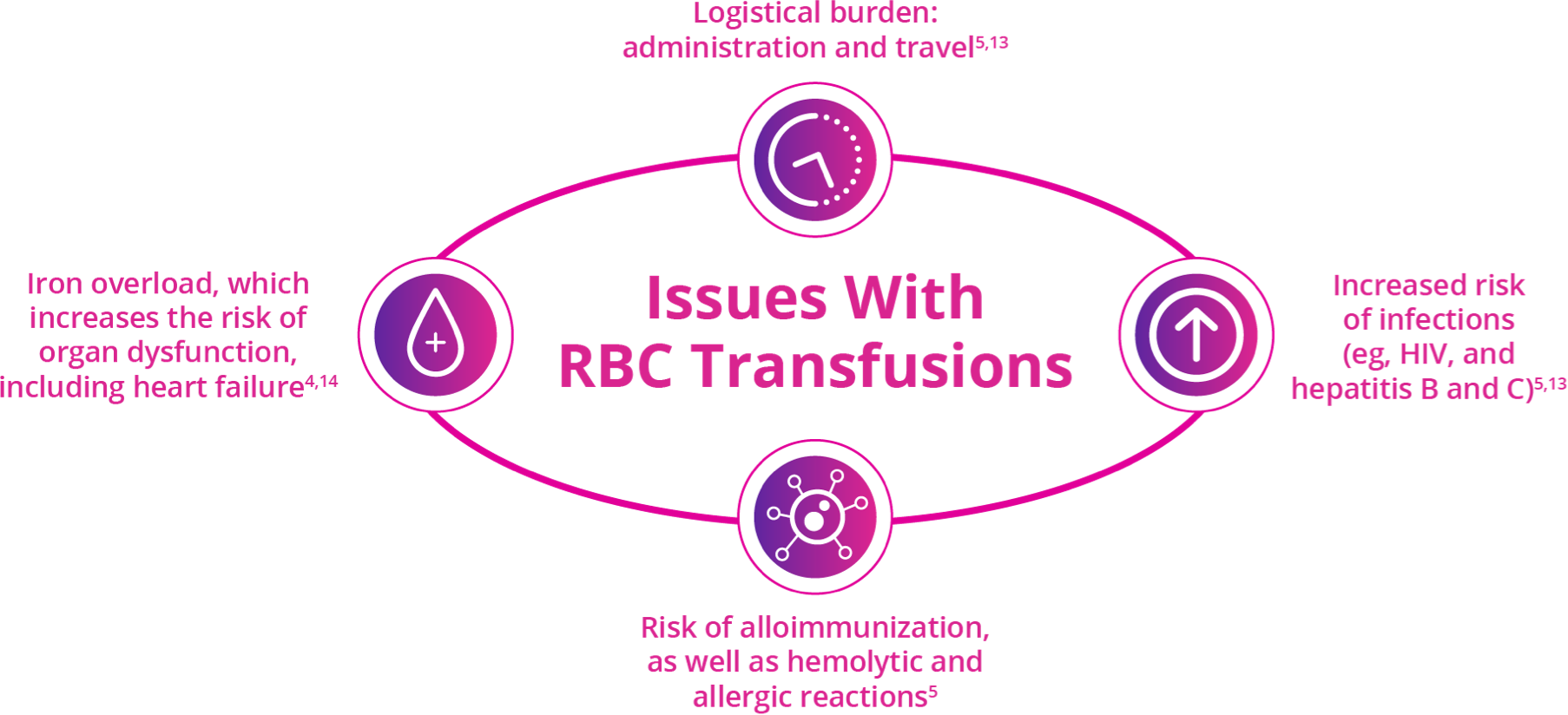
ICT is used for the management of iron overload, which is an inevitable complication of regular blood transfusions because the body lacks a mechanism to excrete excess iron.5
Red blood cell (RBC) transfusions are an important supportive-care option; however, they are burdensome and carry risks2,5,13-15

Due to iron overload, patients with transfusion-dependent
β-thalassemia need to receive iron chelation therapy (ICT)5


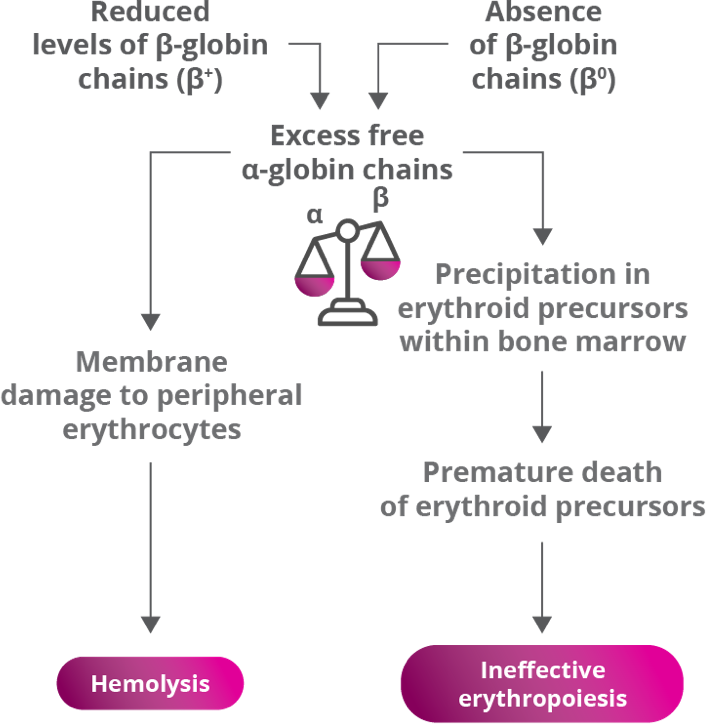
Ineffective erythropoiesis due to mutations in the β-globin gene and hemolysis are hallmarks of β-thalassemia.1-3
β-thalassemia is a rare genetic condition that reduces production of hemoglobin (Hb). It is characterized by reduced or absent synthesis of the β-globin chain of Hb, decreased Hb in the blood, reduced RBC production and anemia.2,4,5
β-globin gene defects lead to reduced (β+) or absent (β0) synthesis of the β chains of hemoglobin and affected patients can have various combinations of normal (β), β+, and/or β0 alleles.2,4,5
β-thalassemia is caused by mutations in the β-globin gene, resulting in ineffective erythropoiesis2,4,5
An estimated 80 million to 90 million people—approximately 1.5% of the global population—are carriers of a β-thalassemia mutation.6
β-thalassemia is most prevalent in the tropics and subtropical regions (see highlighted areas)6,7

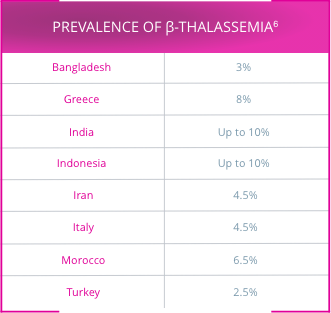
Approximately 200 disease-causing mutations in the β-globin gene have been documented.6 Traditionally, β-thalassemia was classified into 3 main subgroups:8,9
Patients are classified as either transfusion dependent or non-transfusion dependent based on the need for transfusions in order to survive5,9
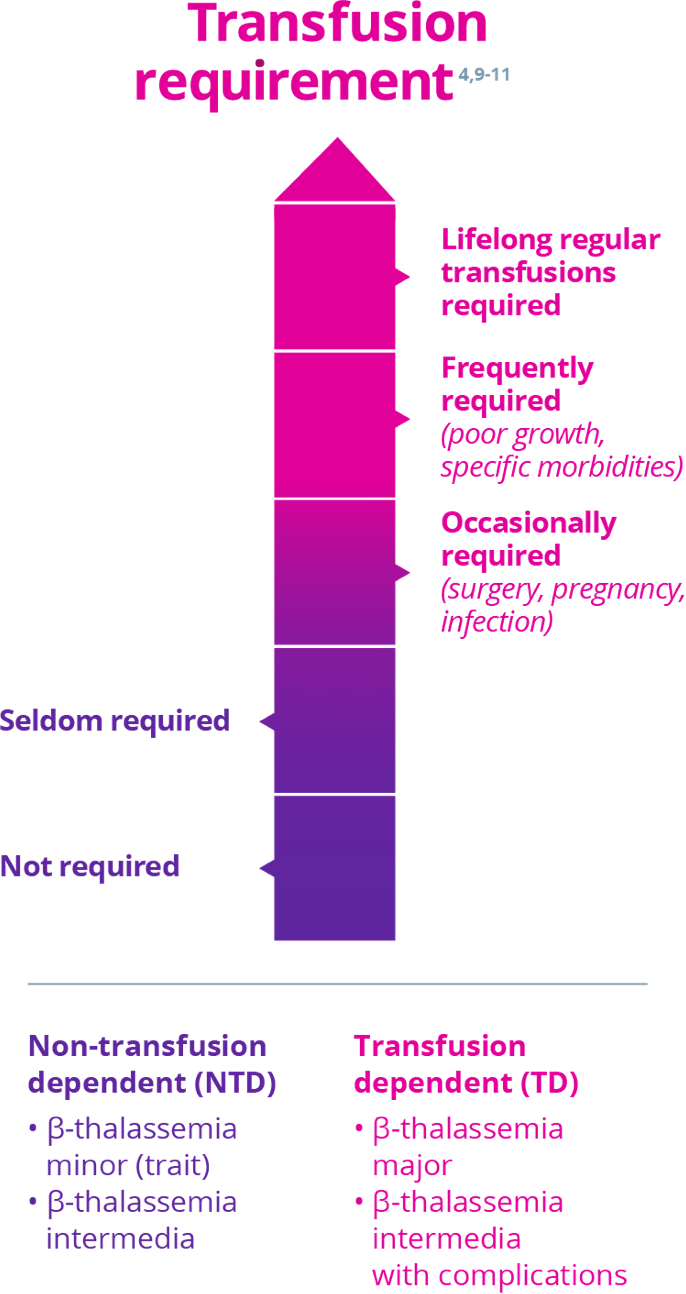
Adapted with permission from Guidelines for the Management of Transfusion Dependent Thalassaemia, 3rd edition, 2014. Reproduction is prohibited.
Current therapy of β-thalassemia is predominantly limited to lifelong red blood cell (RBC) transfusions plus iron chelation therapy (ICT).2,11,12
Red blood cell (RBC) transfusions are an important supportive-care option; however, they are burdensome and carry risks2,5,13-15
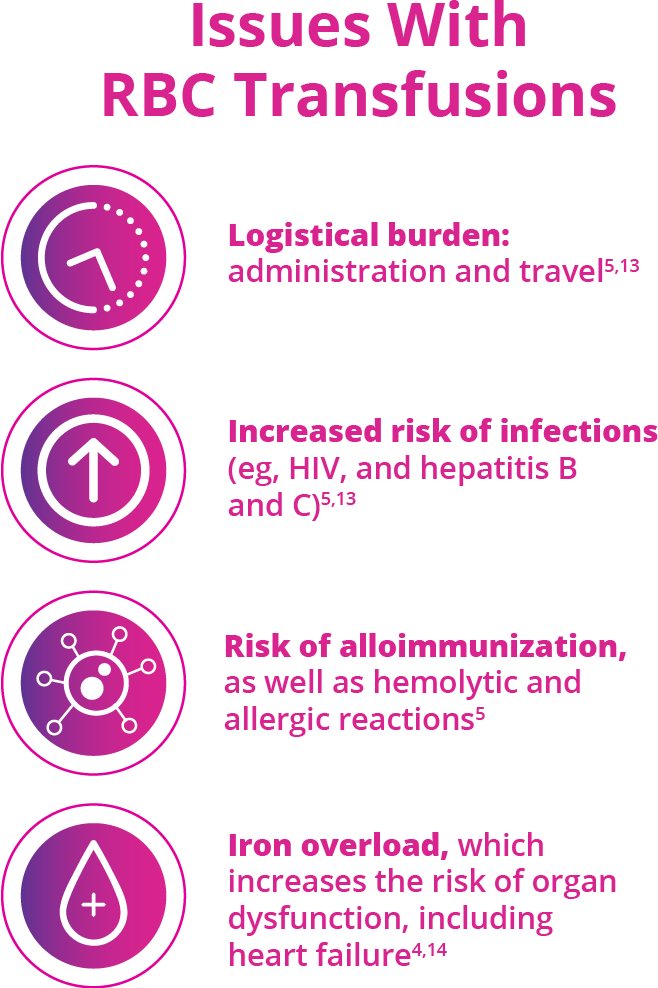
Very few eligible patients receive non-transfusional approaches for managing transfusion-dependent β-thalassemia—such as hematopoietic stem cell transplant or gene therapy.17,18
Due to iron overload, patients with transfusion-dependent
β-thalassemia need to receive iron chelation therapy (ICT)5
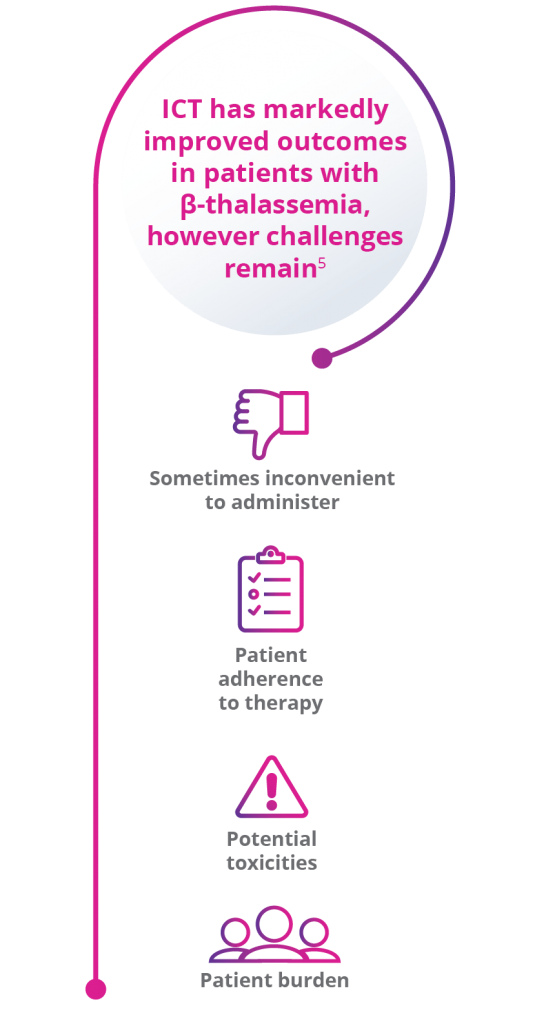
There is a significant unmet need for treatments that could reduce RBC transfusions and related burden in adults with β-thalassemia.11,12