This site is intended for healthcare
professionals in Belgium and Luxembourg.

Erythroid maturation for significant red blood cell (RBC) transfusion reduction
The efficacy of Reblozyl® in β-thalassemia is based on the double-blind, randomized, placebo-controlled, Phase 3 BELIEVE trial (with 224 patients treated with Reblozyl® and 112 patients receiving placebo).1
Reblozyl® significantly reduces RBC transfusion burden.1
Primary Endpoint:
Reblozyl® reduced the RBC transfusion burden by ≥33% and ≥50% during any 12- or 24-week interval.1
Reblozyl® reduces RBC transfusion burden over any consecutive 12- or 24-week interval, a better reflection of assessment in real-world practice than a fixed interval analysis.1,2
Primary Endpoint: ≥33% reduction in RBC transfusion burden
(Weeks 13-24)*1

aP-value from the Cochran Mantel-Haenszel test stratified by the geographical region.1
bCollected over 16 weeks prior to randomization.
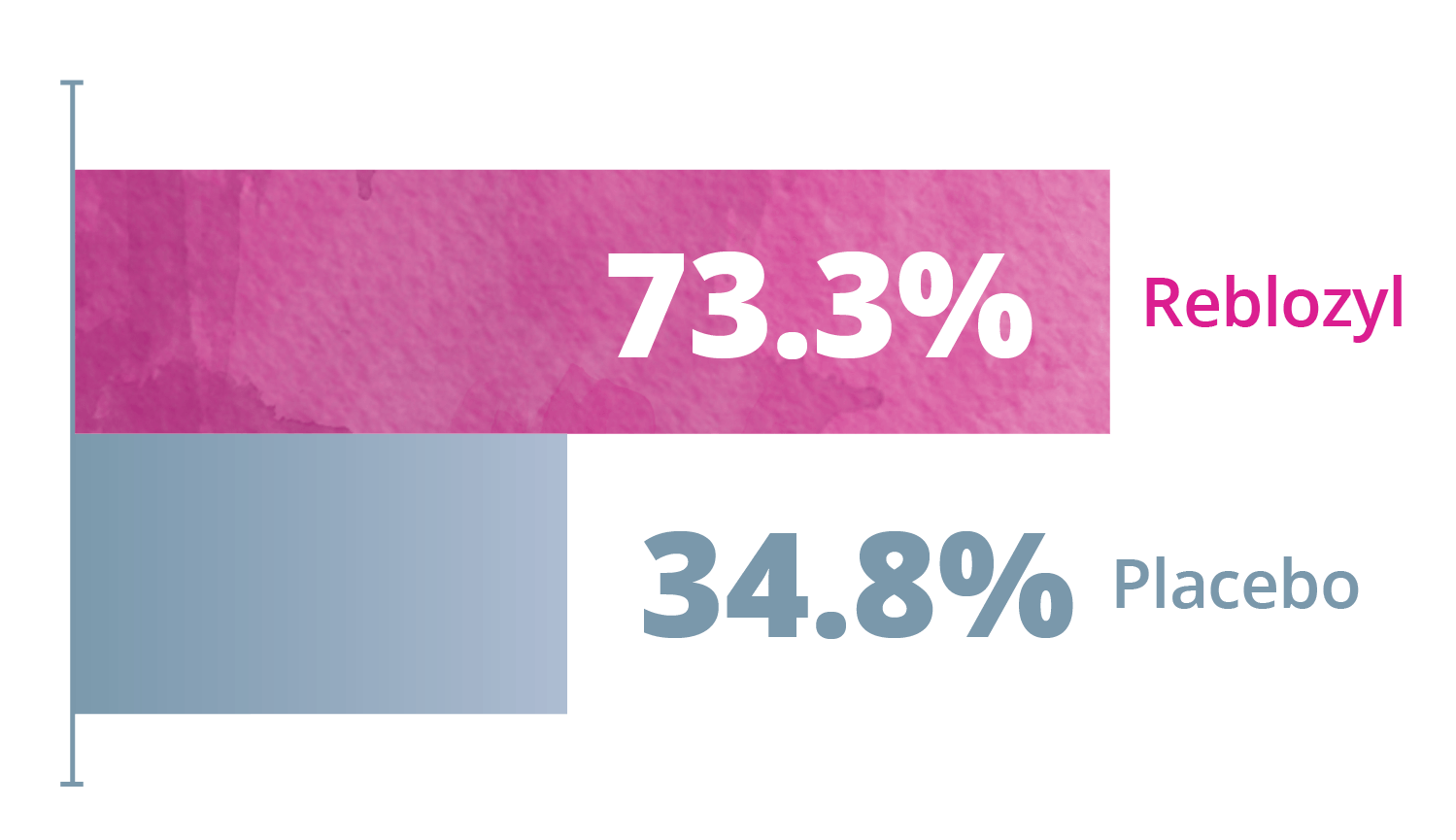
Exploratory Endpoint: ≥33% and ≥50% reduction in any consecutive 12- or 24-week interval*1,3
Any consecutive 12 weeks
Any consecutive 24 weeks
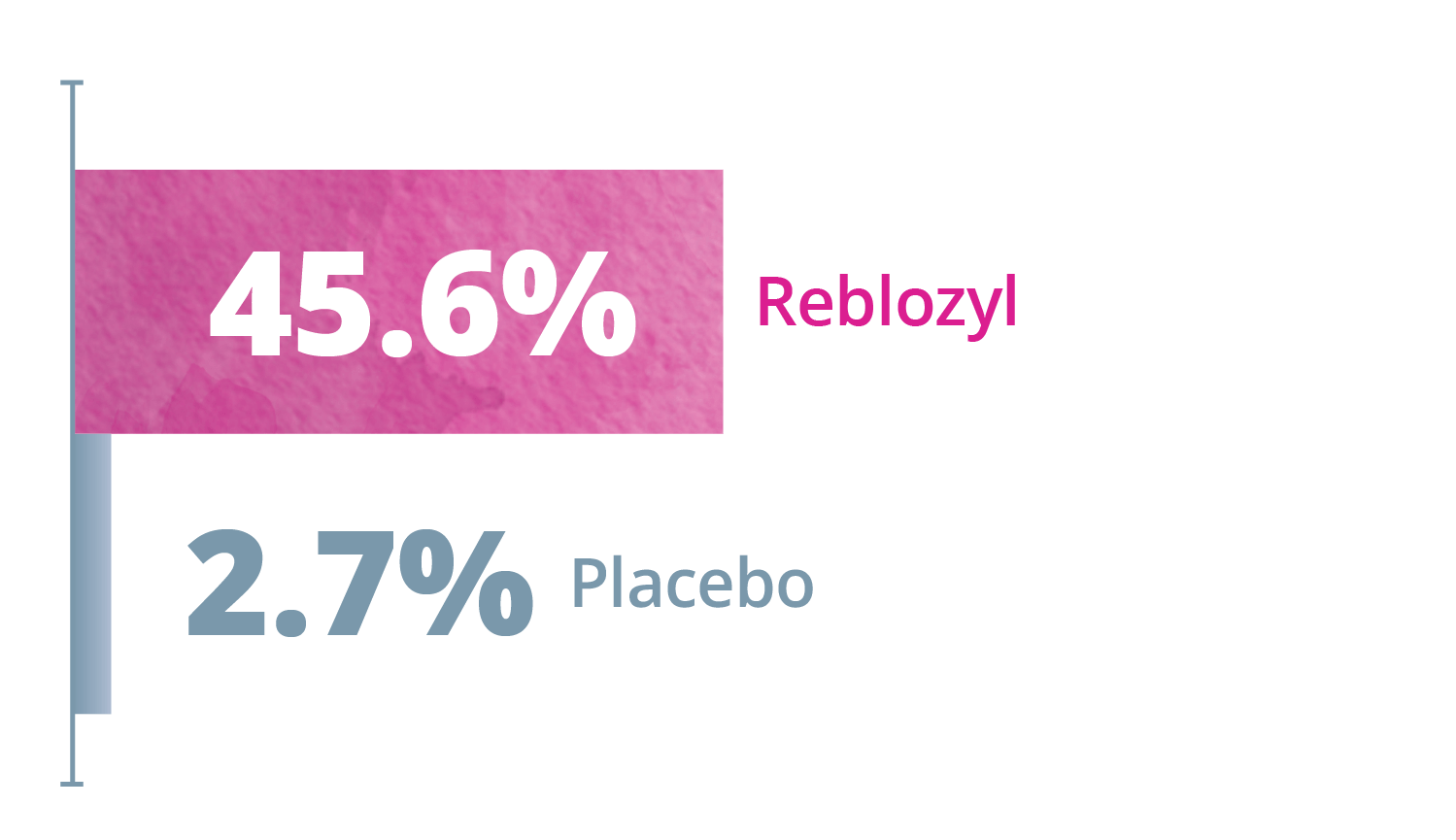
*Reduction from baseline in RBC transfusion burden of at least 2 units for 12 or 24 weeks compared to the 12- or 24-week interval prior to treatment.1

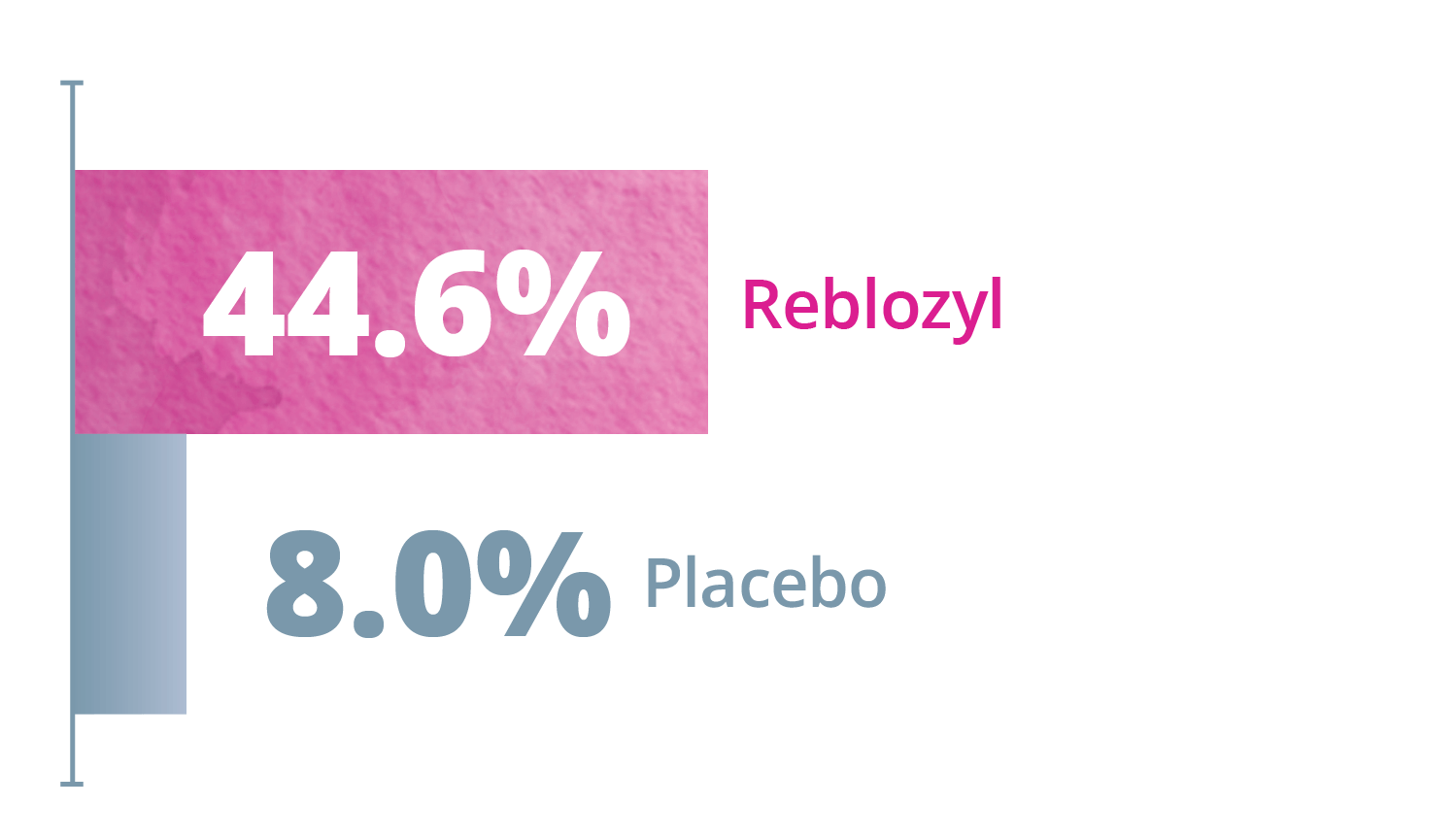
Exploratory Endpoints: ≥50% transfusion reduction for any consecutive 12- or 24 week interval*1,3
Any consecutive 12 weeks
Any consecutive 24 weeks
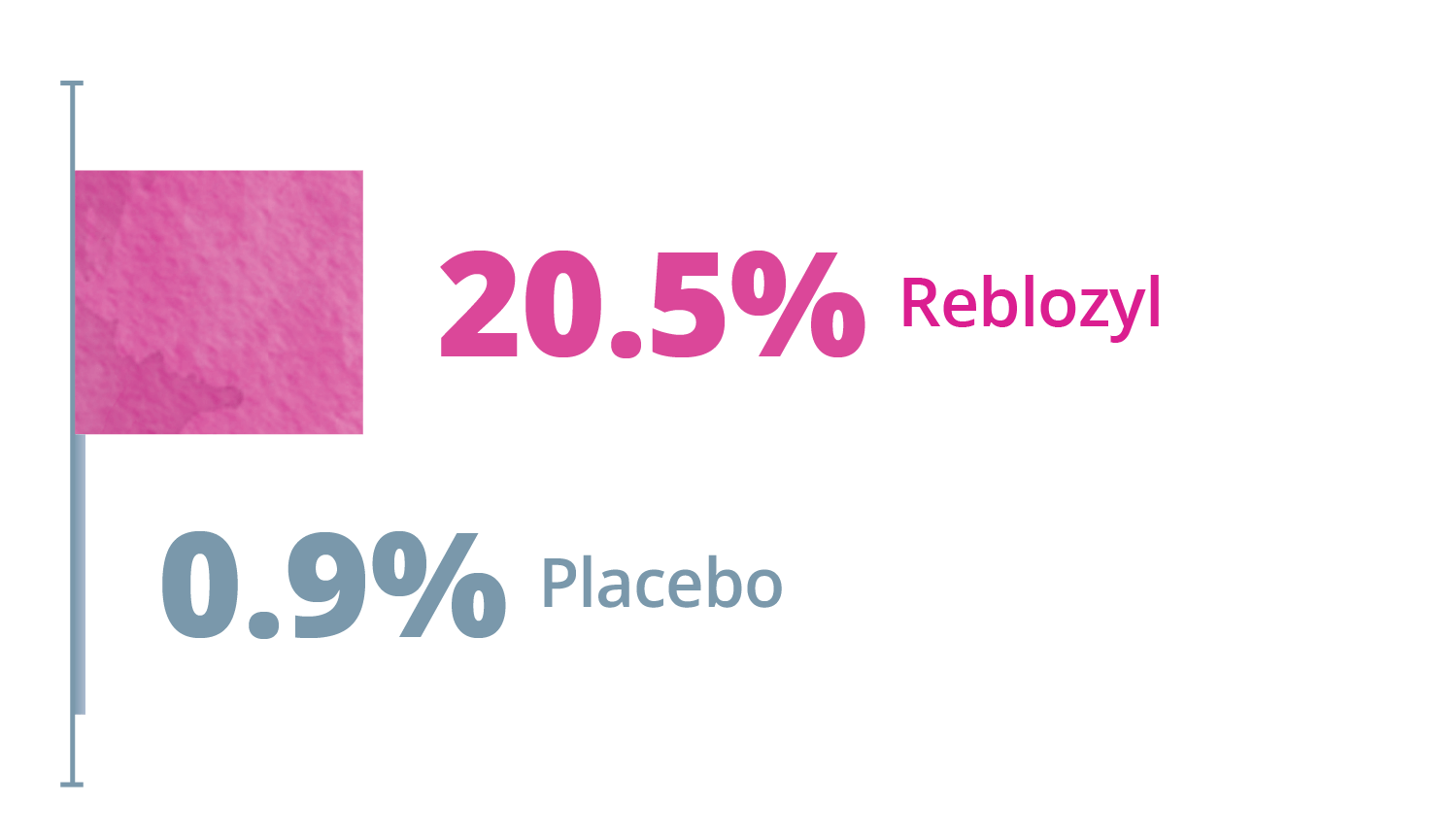
*Reduction from baseline in RBC transfusion burden of at least 2 units for 12 or 24 weeks compared to the 12- or 24-week interval prior to treatment.1
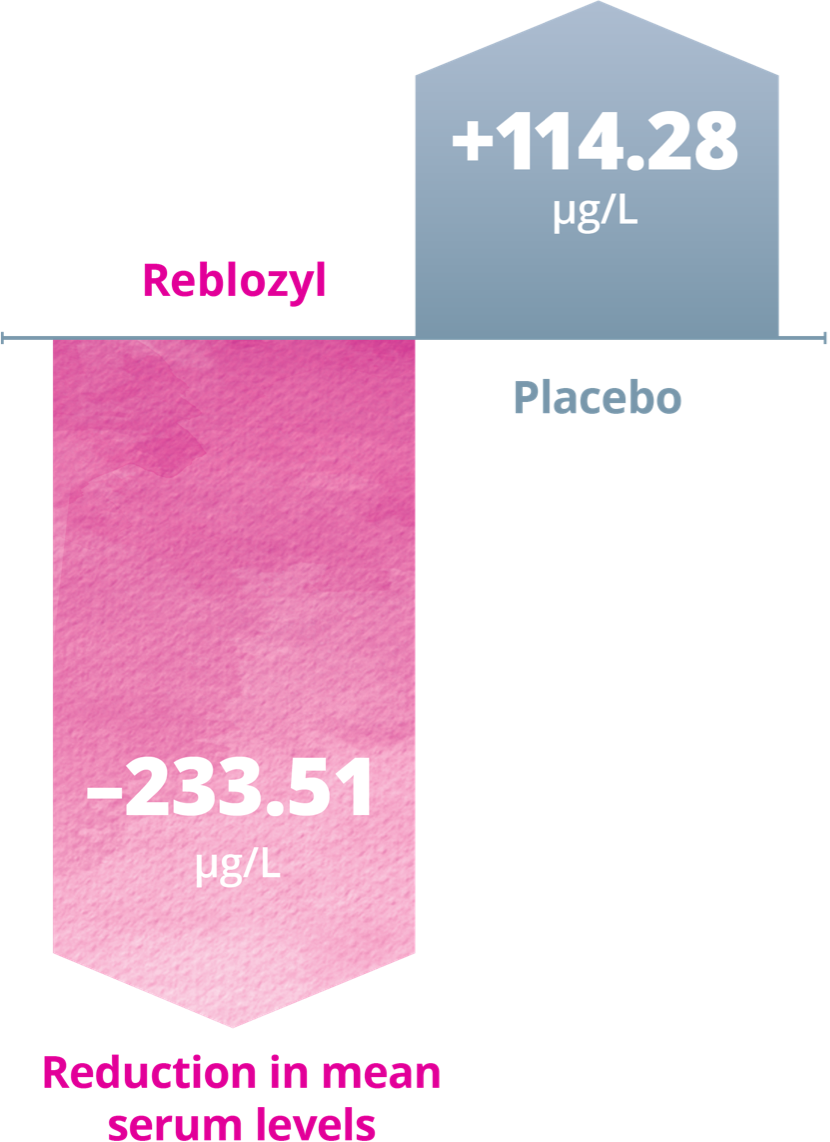
Patients treated with Reblozyl® experienced significant reductions in mean serum ferritin levels at Week 48 vs an increase with placebo1
Impact on serum ferritin levels at Week 481
Erythroid maturation for significant red blood cell (RBC) transfusion reduction
Reblozyl® significantly reduces RBC transfusion burden.1
Primary Endpoint: ≥33% reduction in RBC transfusion burden (Weeks 13-24)*1

aP-value from the Cochran Mantel-Haenszel test stratified by the geographical region.1
*Reduction from baseline in RBC transfusion burden of at least 2 units for 12 consecutive weeks compared to the 12-week interval prior to treatment.1
The efficacy of Reblozyl® in β-thalassemia is based on the double-blind, randomized, placebo-controlled, Phase 3 BELIEVE trial (with 224 patients treated with Reblozyl® and 112 patients receiving placebo).1
Exploratory Endpoint: ≥33% and ≥50% reduction in any consecutive 12- or 24-week interval*1,3
Any consecutive 12 weeks

Any consecutive 24 weeks

*Reduction from baseline in RBC transfusion burden of at least 2 units for 12 or 24 weeks compared to the 12- or 24-week interval prior to treatment.1
Reblozyl® reduces RBC transfusion burden over any consecutive 12- or 24-week interval, a better reflection of assessment in real-world practice than a fixed interval analysis.1,2

†Response assessed during any
consecutive 12-week interval
Exploratory Endpoints: ≥50% transfusion reduction for any consecutive 12- or 24 week interval*1,3
Any consecutive 12 weeks

Any consecutive 24 weeks

*Reduction from baseline in RBC transfusion burden of at least 2 units for 12 or 24 weeks compared to the 12- or 24-week interval prior to treatment.1
Patients receiving Reblozyl® had 4.67 fewer RBC units transfused from Weeks 1-48 versus baseline and 5.66 fewer RBC units transfused from Weeks 49-96, whereas patients under placebo experienced an increase in the RBC units transfused (+1.16 and +2.19, respectively).*1
*Least square mean change from baseline (RBC units/48 weeks).
Patients treated with Reblozyl® experienced significant reductions in mean serum ferritin levels at Week 48 vs an increase with placebo1
Impact on serum ferritin levels at Week 481
