This site is intended for healthcare
professionals in Belgium and Luxembourg.

Reblozyl® should be initiated and monitored under the supervision of a physician experienced in the treatment of hematological diseases.1
START1:
MONITOR1:
INDIVIDUALIZE1:

Dosing with Reblozyl® is customized by patient response. The recommended starting dose of Reblozyl® is 1.0 mg/kg once every 3 weeks.1
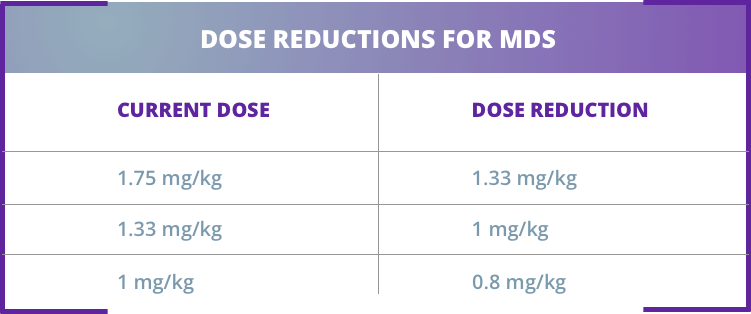
INCREASE DOSE1:
REDUCE DOSE1:
DISCONTINUE1:
Reblozyl® offers stepwise dose increases to achieve
individual patient response1
The dose should not be increased more frequently than every 6 weeks (2 doses) and should not exceed the maximum dose of 1.75 mg/kg every 3 weeks. The dose should not be increased immediately after a dose delay.1

Reconstitution1
Reblozyl® must be reconstituted gently prior to administration. Aggressive shaking should be avoided.
The appropriate number of Reblozyl® vials should be reconstituted to achieve the desired dose. A syringe with appropriate graduations must be used for reconstitution to ensure accurate dosage.
After reconstitution, each mL of solution contains 50 mg of Reblozyl®. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.1
Download the Dosing &
Administration Guides.
These guides include all the information found here, as well as the full steps for reconstitution of Reblozyl®, plus storage information:
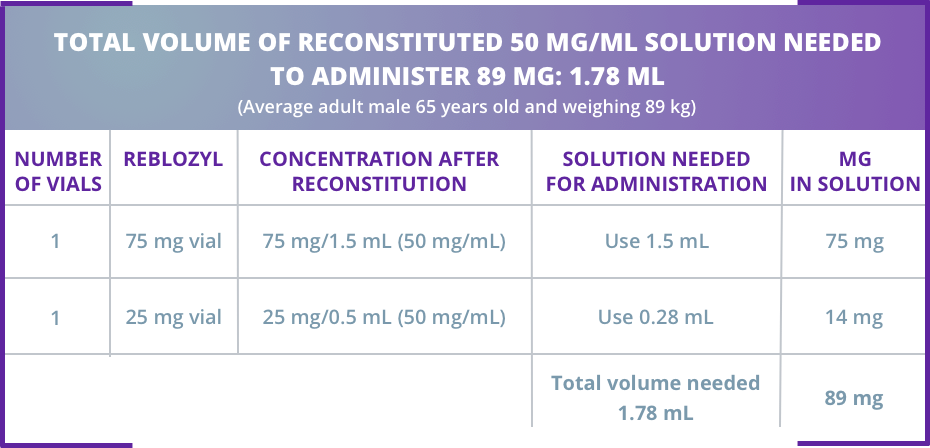
Sample calculation for subcutaneous administration of Reblozyl®
Administering Reblozyl®1
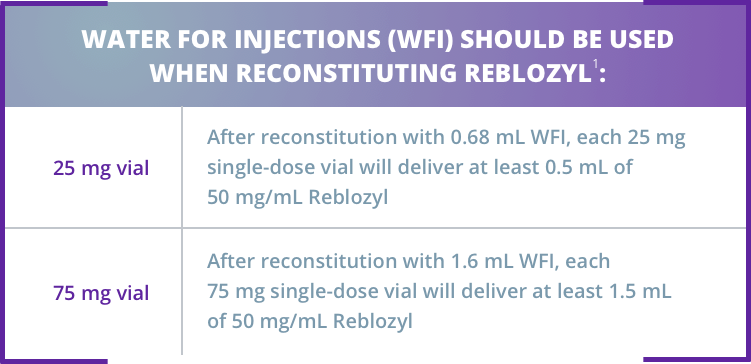
Reblozyl® is available in 2 strengths as single-dose vials for reconstitution1
Reblozyl® is supplied as a lyophilized powder for reconstitution before use
Sample calculation for subcutaneous administration of Reblozyl®
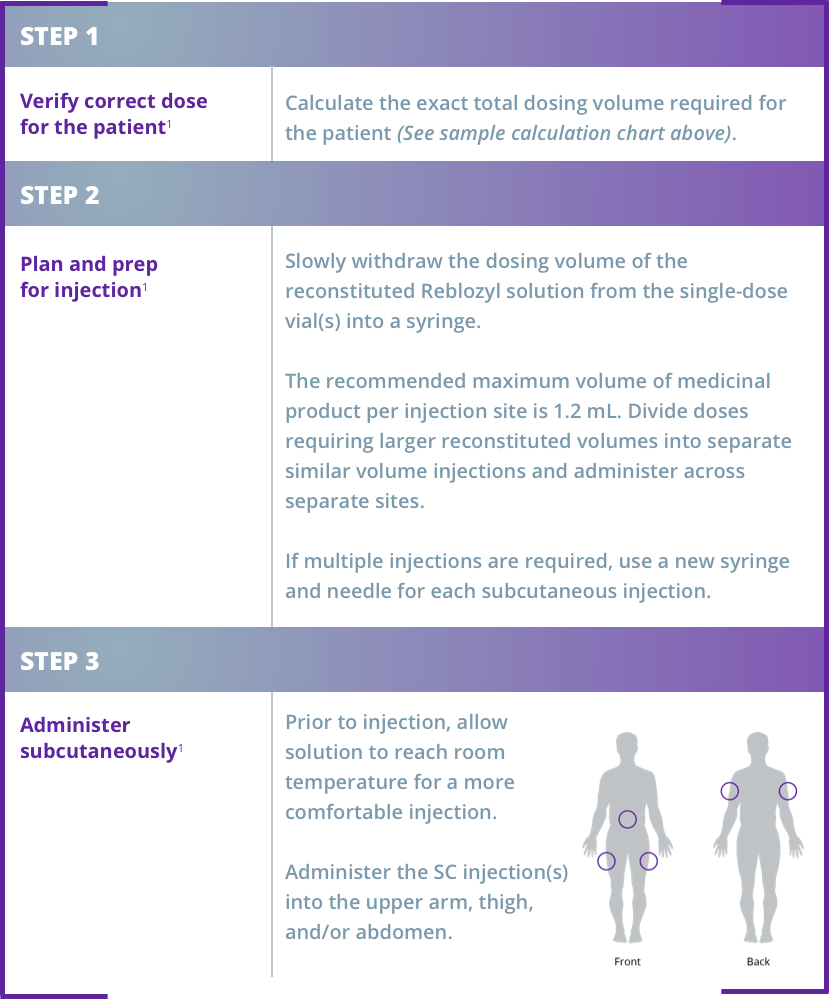
Dividing doses and administering with reconstituted volumes larger than 1.2 mL:1
Injection 1: 0.89 mL – in upper arm Injection 2: 0.89 mL – in thigh or abdomen
Reblozyl® is administered subcutaneously every 3 weeks1
Do not administer more than 1 dose from a vial
Do not mix with other medications
Do not pool unused portions from the vials
Discard any unused portion
Reblozyl® should be initiated and monitored under the supervision of a physician experienced in the treatment of hematological diseases.1
Dosing with Reblozyl® is customized by patient response. The recommended starting dose of Reblozyl® is 1.0 mg/kg once every 3 weeks.1
Reblozyl® offers stepwise dose increases to achieve individual patient response1
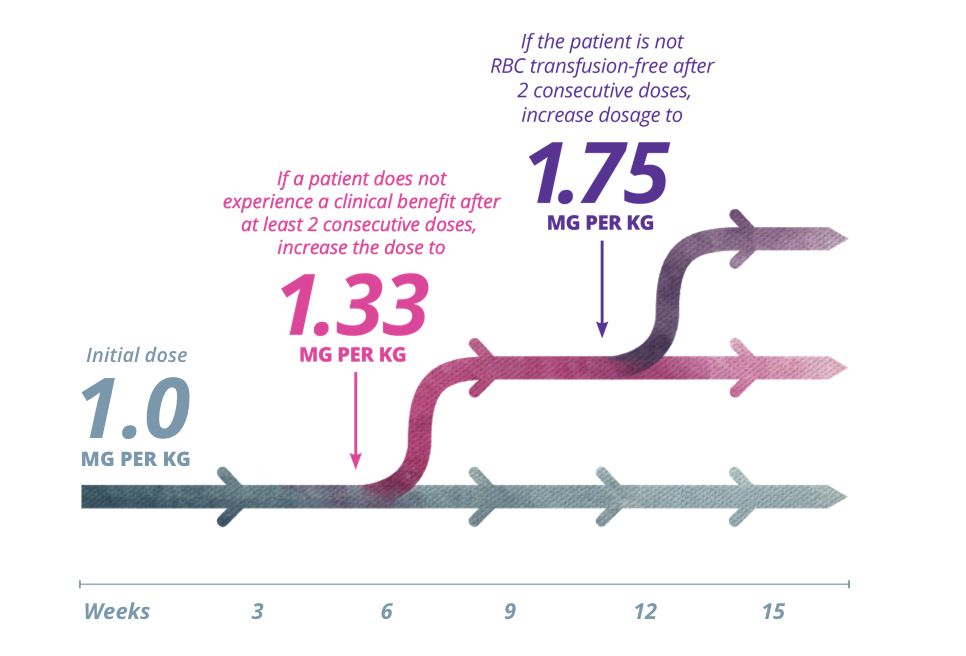
The dose should not be increased more frequently than every 6 weeks (2 doses) and should not exceed the maximum dose of 1.75 mg/kg every 3 weeks. The dose should not be increased immediately after a dose delay.1

Reblozyl® is available in 2 strengths as single-dose vials for reconstitution1

Reblozyl® must be reconstituted gently prior to administration. Aggressive shaking should be avoided.
The appropriate number of Reblozyl® vials should be reconstituted to achieve the desired dose. A syringe with appropriate graduations must be used for reconstitution to ensure accurate dosage.
After reconstitution, each mL of solution contains 50 mg of Reblozyl®. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.1
Download the Dosing & Administration Guides.
These guides include all the information found here, as well as the full steps for reconstitution of Reblozyl®, plus storage information:
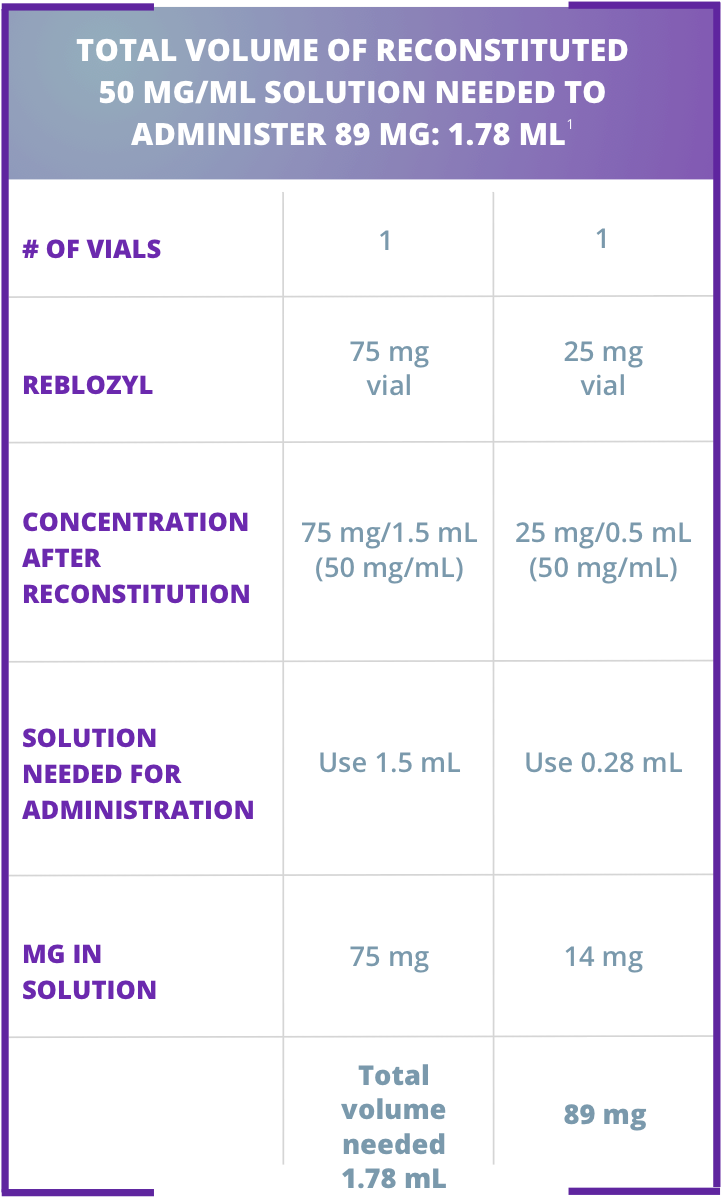
Dividing doses and administering with reconstituted volumes larger than 1.2 mL:1
Injection 1: 0.89 mL – in upper arm
Injection 2: 0.89 mL – in thigh or abdomen
Reblozyl® is administered subcutaneously every 3 weeks1

Do not administer more than 1 dose from a vial
Do not mix with other medications
Do not pool unused portions from the vials
Discard any unused portion