This site is intended for healthcare
professionals in Belgium and Luxembourg.

The efficacy of Reblozyl® in MDS is based on the double-blind, randomized, placebo-controlled, phase 3 MEDALIST trial (with 153 patients treated with Reblozyl® and 76 patients receiving placebo).1
Significantly more patients experienced RBC transfusion independence (TI) with Reblozyl® vs placebo. 1
Significantly more patients on Reblozyl®experienced a longer RBC-TI period vs placebo. 1
Patients receiving Reblozyl® had fewer RBC transfusion events vs placebo during Weeks 1-24 and Weeks 25-48 1
During Weeks 1-24:
During Weeks 25-48:
Patients receiving Reblozyl® required fewer RBC transfusion units vs placebo during Weeks 1-24 and Weeks 25-48 1
During Weeks 1-24:
During Weeks 25-48:
Primary Endpoint: RBC transfusion independence (TI)
≥8 weeks • Weeks 1-241
Significantly more patients experienced RBC-TI with Reblozyl® vs placebo

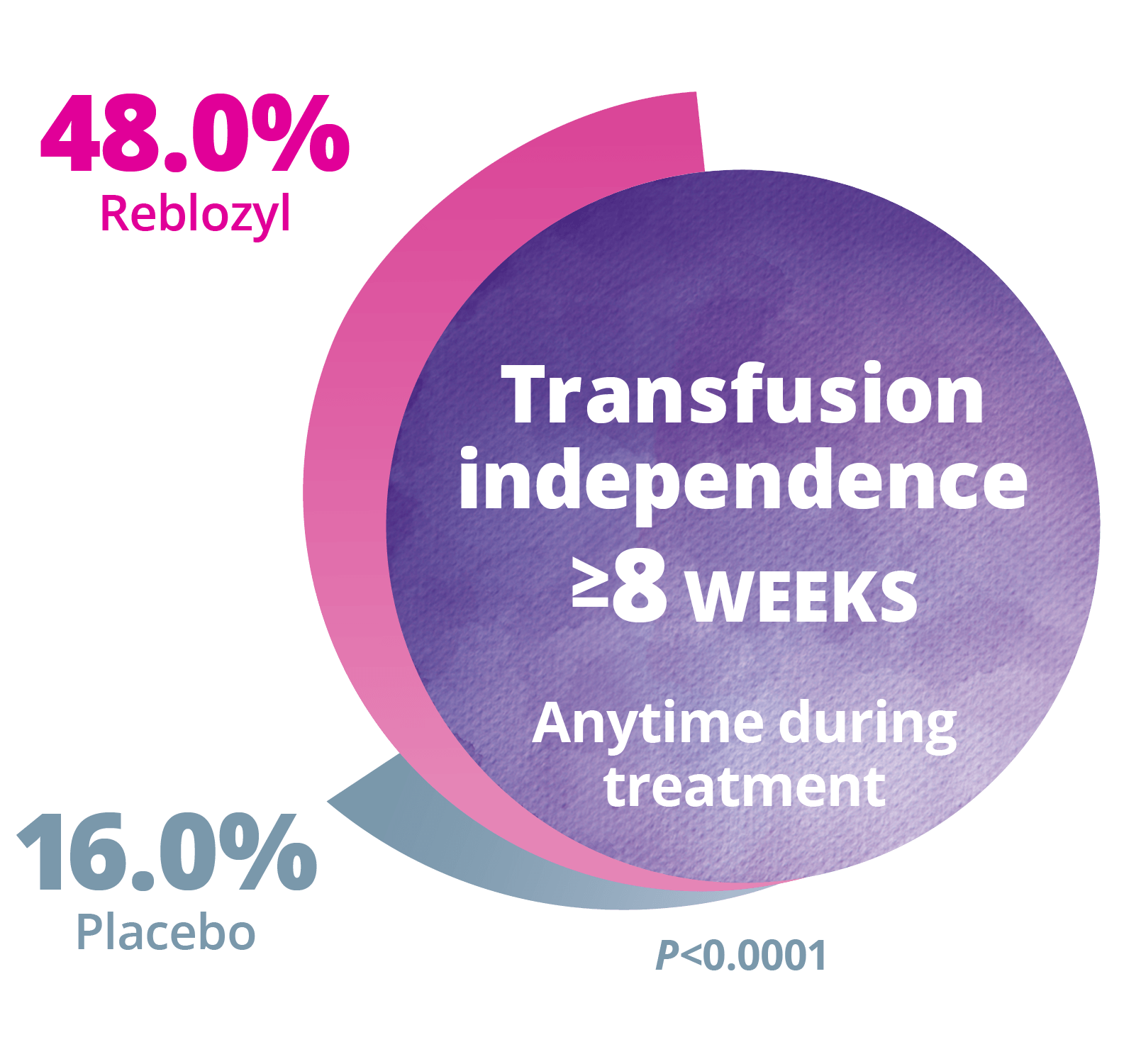
Longer term Follow-up: RBC transfusion independence
≥8 weeks (over the entire period of treatment)2
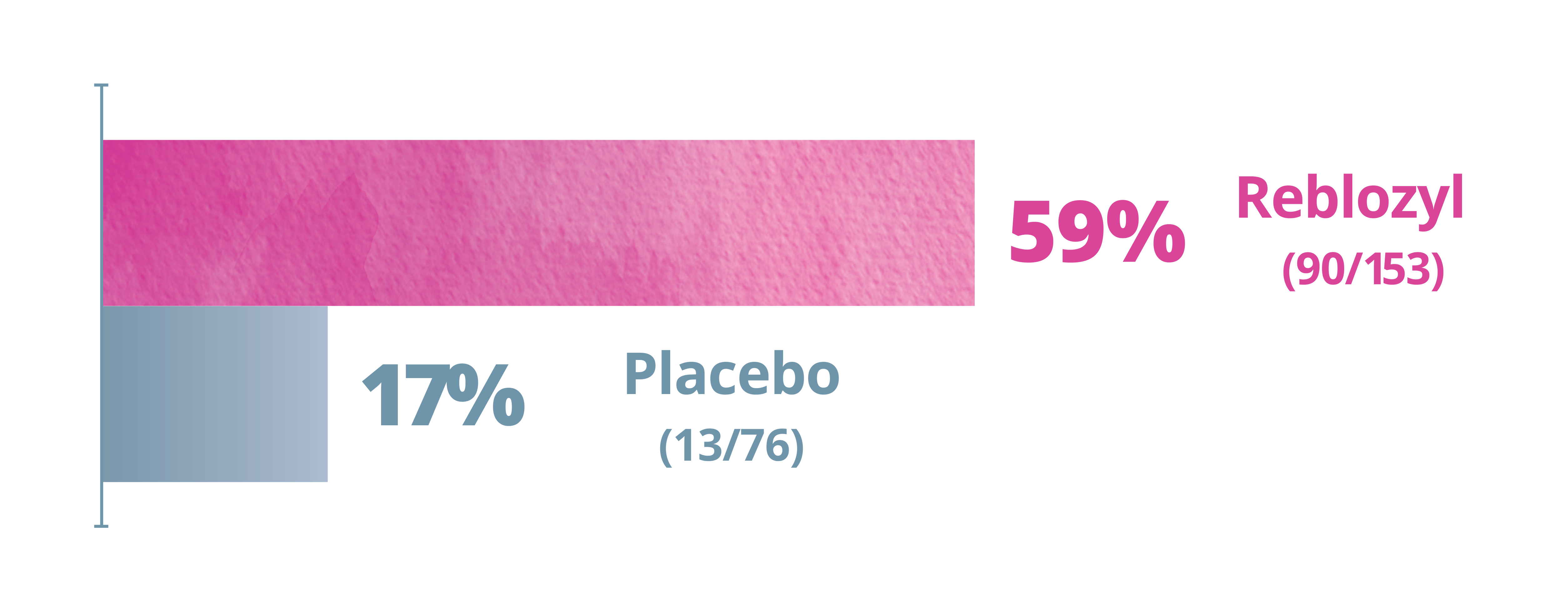
Key Secondary Endpoint: RBC-TI ≥12 weeks • Weeks 1-481
Significantly more patients experienced a longer RBC-TI period with Reblozyl® vs placebo

Key Secondary Endpoint: RBC transfusion event frequency •
Weeks 1-241

Key Secondary Endpoint: RBC transfusion units • Weeks 1-241

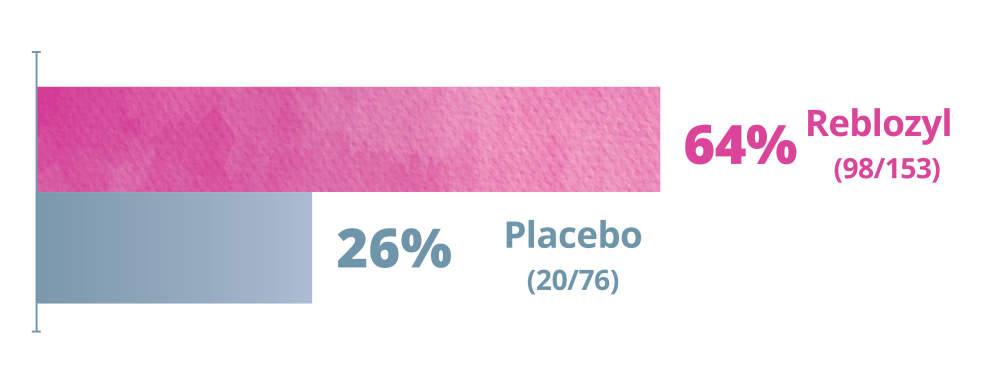
More patients receiving Reblozyl® achieved a hematologic improvement through Week 48 vs placebo1
During Weeks 1-48:
Exploratory Endpoint: Modified hematologic improvement-erythroid (mHI-E)a,b,c Weeks 1-481
More patients achieved mHI-E at Week 48 with Reblozyl® vs placebo
aResponse criteria were developed by the IWG.
bThe proportion of patients meeting the HI-E criteria as per IWG 2006 criteria sustained over a consecutive 56-day period during the indicated treatment period.
cIn patients with a baseline transfusion burden of ≥4 units/8 weeks, mHI-E was defined as a reduction in RBC transfusions of at least 4 units/8 weeks. For patients with a baseline RBC burden of <4 units/8 weeks, mHI-E was defined as a mean increase in hemoglobin of ≥1.5 g/dL for 8 weeks in the absence of transfusions.
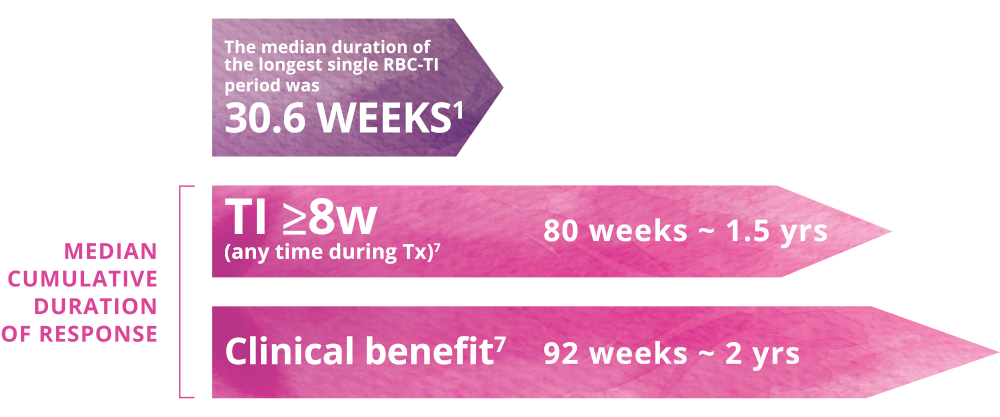
More patients receiving Reblozyl® achieved a clinical benefit over the entire treatment period.
Clinical Benefit over the entire treatment period
Response to Reblozyl® treatment was rapid and sustained.1
Reblozyl® treatment response
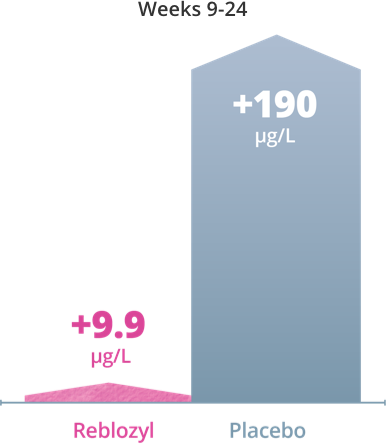
Lower mean change in serum ferritin levels were observed from baseline with Reblozyl® compared with placebo.1
Exploratory Endpoint: Impact on serum ferritin levels with Reblozyl®1
Erythroid maturation for significant red blood cell (RBC) transfusion reduction
Primary Endpoint: RBC transfusion
independence (TI) ≥8 weeks •
Weeks 1-241
Significantly more patients experienced RBC-TI with Reblozyl® vs placebo

Longer term Follow-up: RBC transfusion independence ≥8 weeks (over the entire period of treatment)2
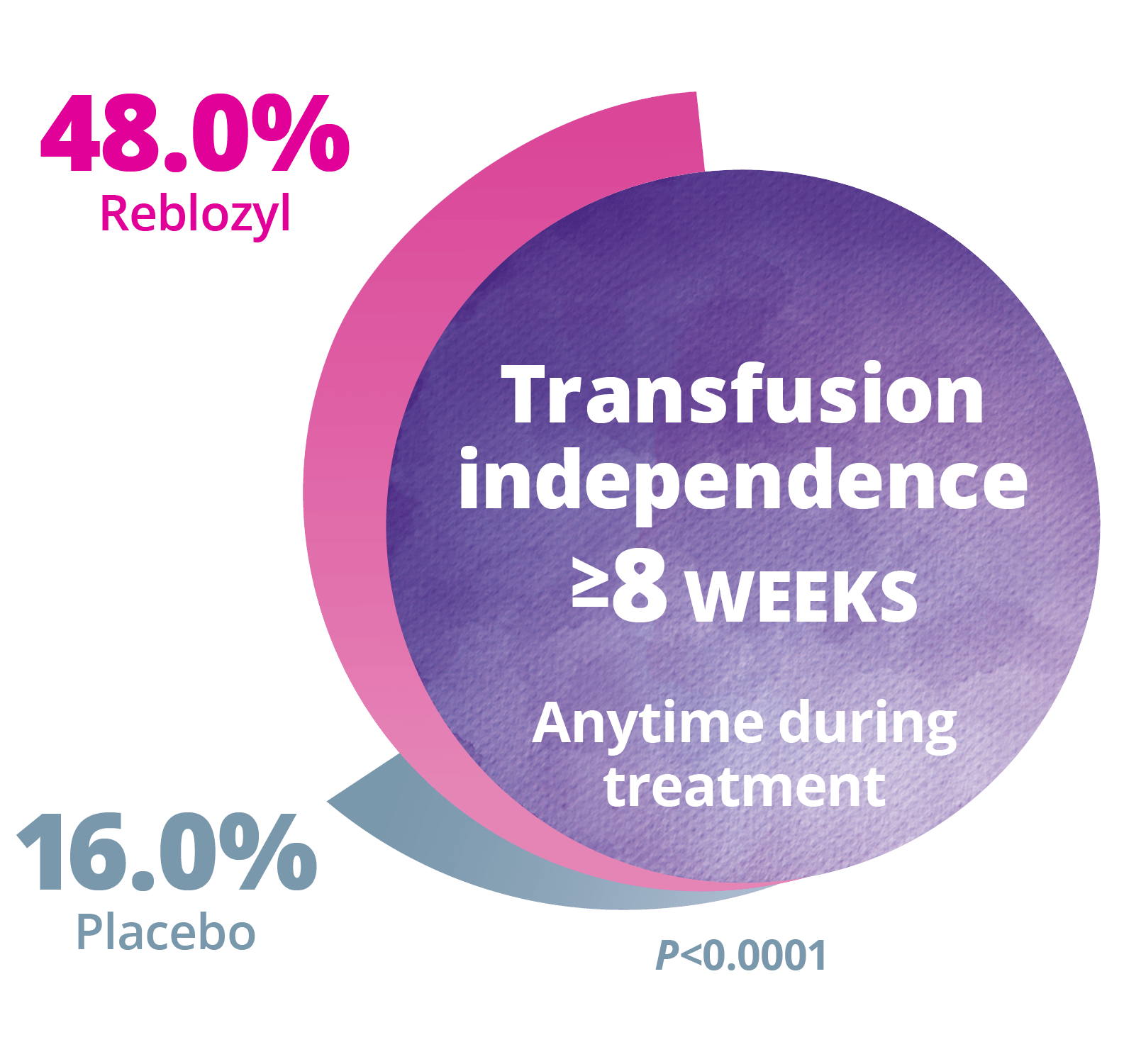
The efficacy of Reblozyl® in MDS is based on the double-blind, randomized, placebo-controlled, phase 3 MEDALIST trial (with 153 patients treated with Reblozyl® and 76 patients receiving placebo).1
Key Secondary Endpoint: RBC-TI ≥12 weeks • Weeks 1-481
Significantly more patients experienced a longer RBC-TI period with Reblozyl® vs placebo

Key Secondary Endpoint: RBC transfusion event frequency • Weeks 1-241
Patients receiving Reblozyl® had fewer RBC transfusion events vs placebo during Weeks 1-24 and Weeks 25-481

During Weeks 1-24:
During Weeks 25-48:
Key Secondary Endpoint: RBC transfusion units • Weeks 1-241
Patients receiving Reblozyl® required fewer RBC transfusion units vs placebo during Weeks 1-24 and Weeks 25-481
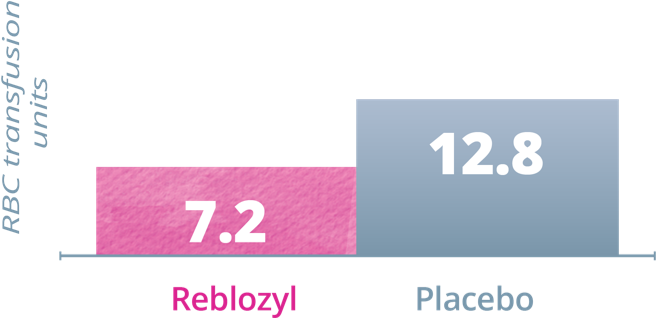
Low baseline transfusion burden (<6 units/8 weeks)
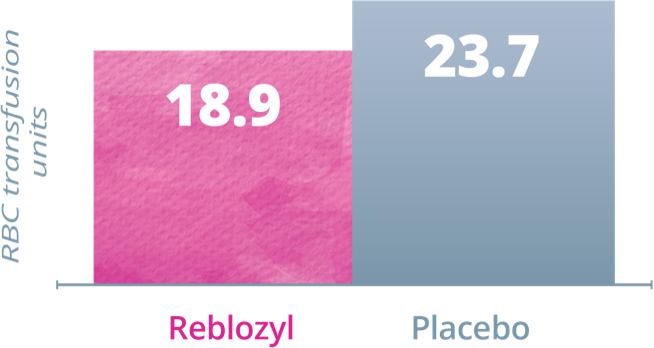
High baseline transfusion burden (≥6 units/8 weeks)
During Weeks 1-24:
During Weeks 25-48:
Erythroid maturation for achieving hematologic improvement
More patients on Reblozyl® achieved a hematologic improvement, according to the International Working Group (IWG) 2006 response criteria, during Weeks 1-24.
Exploratory Endpoint: Modified hematologic improvement-erythroid (mHI-E)a,b,c Weeks 1-481
More patients achieved mHI-E at Week 48 with Reblozyl® vs placebo

aResponse criteria were developed by the IWG.
bThe proportion of patients meeting the HI-E criteria as per IWG 2006 criteria sustained over a consecutive 56-day period during the indicated treatment period.
cIn patients with a baseline transfusion burden of ≥4 units/8 weeks, mHI-E was defined as a reduction in RBC transfusions of at least 4 units/8 weeks. For patients with a baseline RBC burden of <4 units/8 weeks, mHI-E was defined as a mean increase in hemoglobin of ≥1.5 g/dL for 8 weeks in the absence of transfusions.
More patients receiving Reblozyl® achieved a clinical benefit over the entire treatment period.
Clinical Benefit over the entire treatment period

Response to Reblozyl® treatment was rapid and sustained.
Reblozyl® treatment response

Lower mean change in serum ferritin levels were observed from baseline with Reblozyl® compared with placebo.1
Exploratory Endpoint: Impact on serum ferritin levels with Reblozyl®1
