This site is intended for healthcare
professionals in Belgium and Luxembourg.

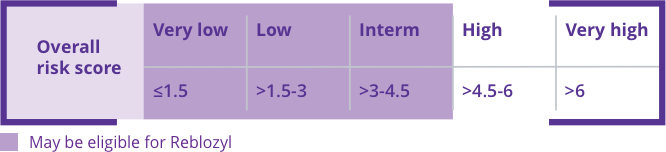
The IPSS-R provides a more accurate prognosis in terms of overall survival and evolution to acute myeloid leukemia compared with the IPSS.2
The IPSS-R accounts for:
The International Prognosis Scoring System-Revised (IPSS-R) helps identify very low- to intermediate-risk patients who may be eligible for Reblozyl®.1,2

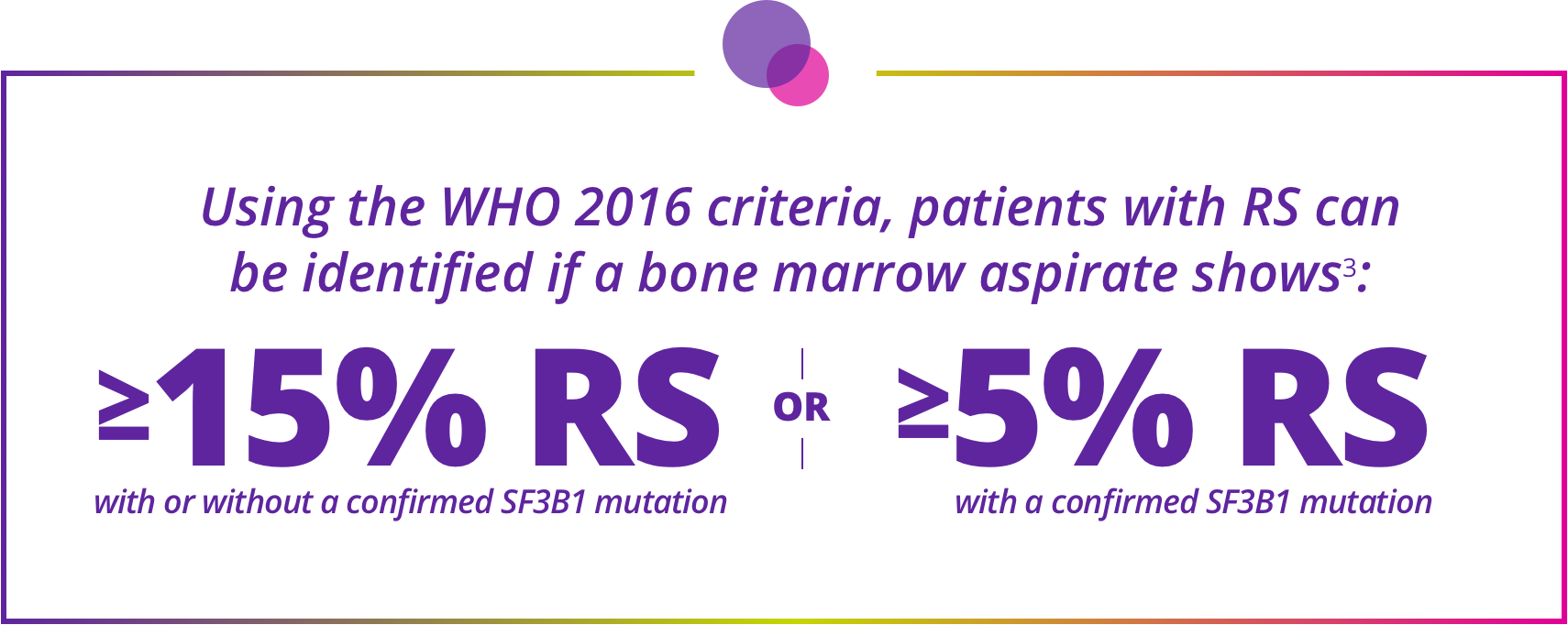
Staining for RS and checking for SF3B1 mutations are needed to accurately classify MDS using WHO 2016 criteria.3
Determining ring sideroblast (RS) status is essential for accurate classification of MDS subtypes and patient eligibility for Reblozyl®3
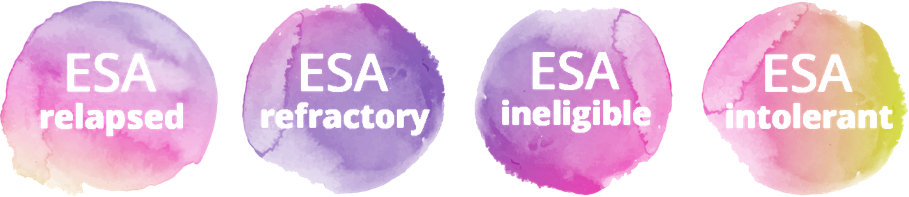
There is an unmet clinical need for patients who have an unsatisfactory response to or are ineligible for erythropoiesis-stimulating agents (ESAs)7-8
Primary ESA resistance is more frequent among patients with MDS-RS vs MDS without RS, while durable responses are less frequent9,12

Pregnancy
Treatment with Reblozyl® should not be started if the woman is pregnant. There are no data from the use of Reblozyl® in pregnant women. Studies in animals have shown reproductive toxicity.
Women of childbearing potential should use effective contraception during treatment with Reblozyl® and for at least 3 months after the last dose.
Prior to starting treatment with Reblozyl®, a pregnancy test should be performed for women of childbearing potential.
If a patient becomes pregnant, Reblozyl® should be discontinued.
Contraindications1
PREGNANCY
HYPERSENSITIVITY
to the active substance or to any of the excipients
Please consult the full summary of Product Characteristics (SmPC) before prescribing: SmPC in French, SmPC in Dutch.
Traceability
In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Increased blood pressure
In controlled clinical studies in MDS and β-thalassemia, patients treated with Reblozyl® had an average increase in systolic and diastolic blood pressure of 5 mm Hg from baseline not observed in patients receiving placebo. Blood pressure should be monitored prior to each Reblozyl® administration. In the case of persistent hypertension or exacerbations of pre-existing hypertension, patients should be treated for hypertension as per current clinical guidelines.
Sodium content
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, that is to say it is essentially “sodium-free.”
TRACEABILITY
Record the name and batch number of Reblozyl®
THROMBOEMBOLIC EVENTS
The occurrence of TEE was not correlated with elevated
hemoglobin levels
INCREASED BLOOD PRESSURE
Monitor blood pressure prior to administering Reblozyl®
SODIUM CONTENT
Reblozyl® is essentially “sodium-free”
The International Prognosis Scoring System-Revised (IPSS-R) helps identify very low- to intermediate-risk patients who may be eligible for Reblozyl®.1,2
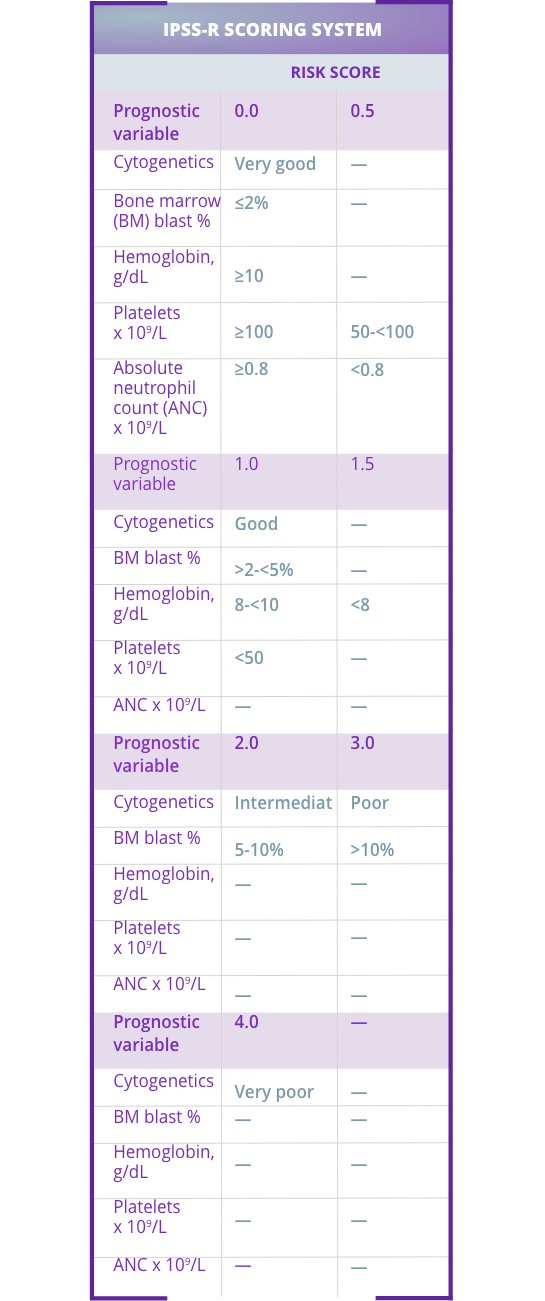
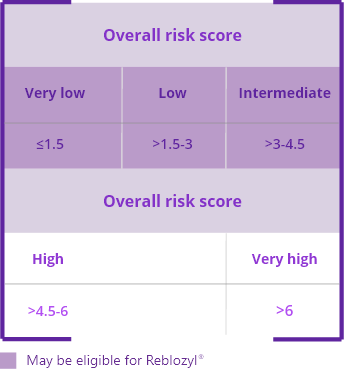
The IPSS-R provides a more accurate prognosis in terms of overall survival and evolution to acute myeloid leukemia compared with the IPSS.2
The IPSS-R accounts for:
Determining ring sideroblast (RS) status is essential for accurate classification of MDS subtypes and patient eligibility for Reblozyl®3
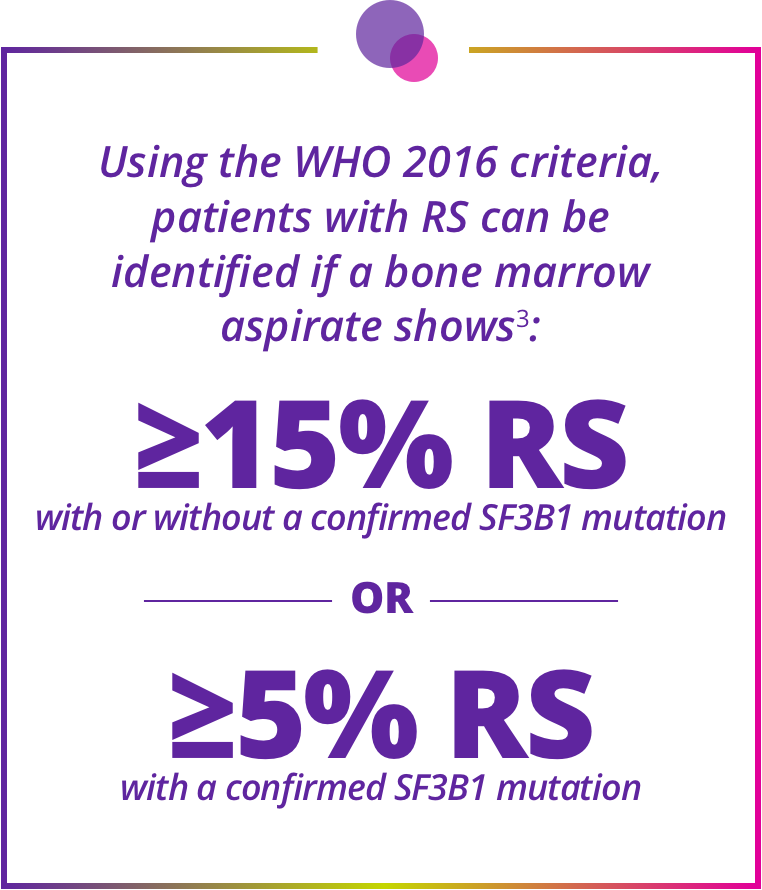
Staining for RS and checking for SF3B1 mutations are needed to accurately classify MDS using WHO 2016 criteria.
There is an unmet clinical need for patients who have an unsatisfactory response to or are ineligible for erythropoiesis-stimulating agents (ESAs)7-8
Contraindications1
Pregnancy
Treatment with Reblozyl® should not be started if the woman is pregnant. There are no data from the use of Reblozyl® in pregnant women. Studies in animals have shown reproductive toxicity.
Women of childbearing potential should use effective contraception during treatment with Reblozyl® and for at least 3 months after the last dose.
Prior to starting treatment with Reblozyl®, a pregnancy test should be performed for women of childbearing potential.
If a patient becomes pregnant, Reblozyl® should be discontinued.
Please consult the full summary of Product Characteristics (SmPC) before prescribing: SmPC in French, SmPC in Dutch.